Infrastructure
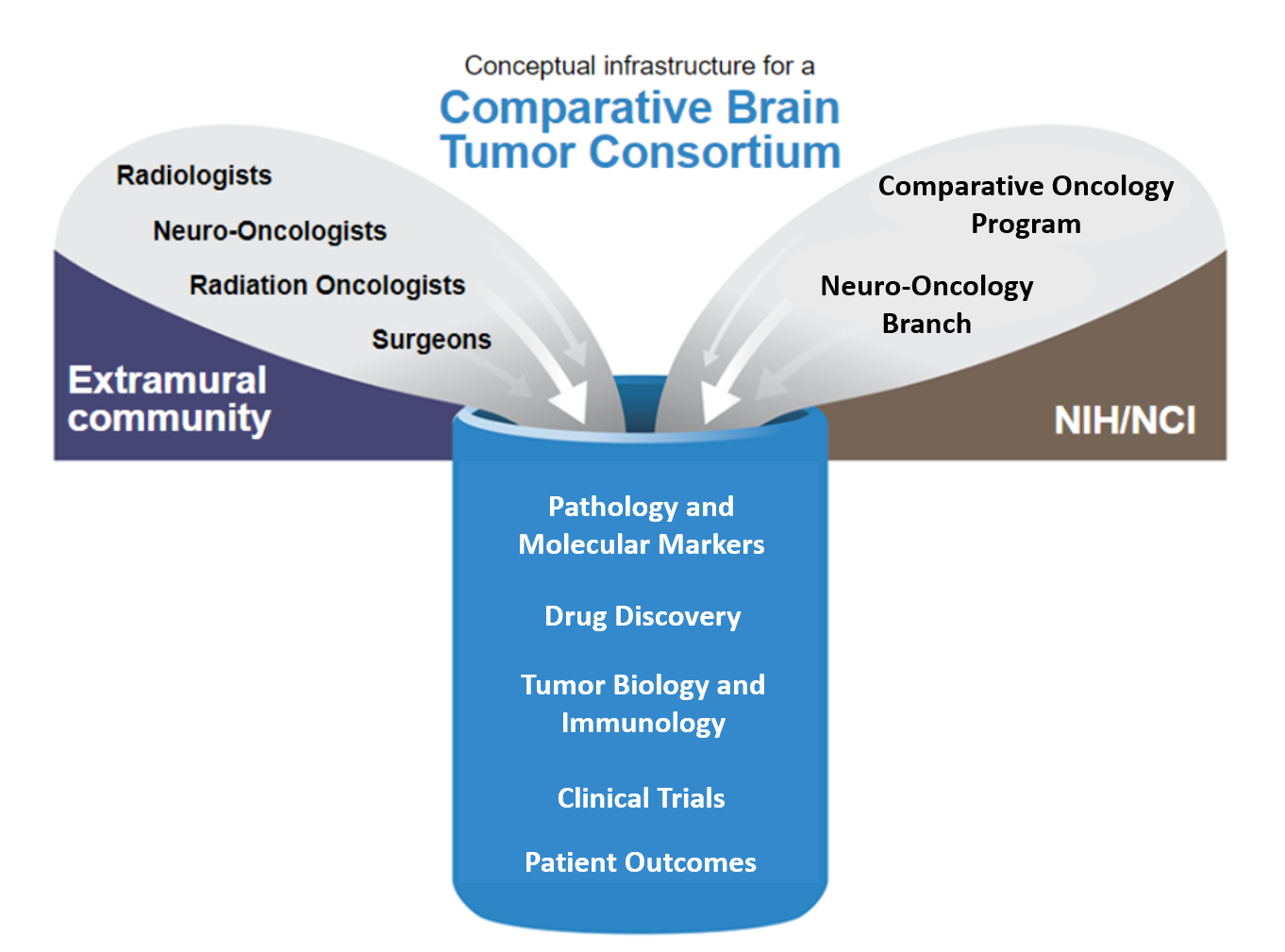
The infrastructure of the Comparative Brain Tumor Consortium includes radiologists, neuro-oncologists, radiation oncologists and surgeons from the extramural community as well as staff from the NCI's Comparative Oncology Program and Neuro-Oncology Branch. Learn more about the consortium's activities below.
One of the key goals of the Comparative Brain Tumor Consortium (CBTC) is to establish the translational relevance of canine brain tumors as models for their human counterparts. To begin to address this highly complex and involved project, we began by investigating the histologic landscape of canine glioma and engaging a panel of expert physician and veterinary neuropathologists. The goal is to evaluate the features of canine glioma and determine how best to classify them with input from both human and veterinary perspectives, so that common ground can be established at the most basic level of tumor diagnosis.
This pathology board convened on March 20-21, 2017 to review and propose a new classification schema for canine glioma. The board consisted of 6 MD pathologists and 10 DVM pathologists, and collectively is reviewing over 200 canine cases collected from 11 different veterinary institutions.
Future efforts in this project will include review of existing and planned molecular phenotyping datasets that can be correlated back to histologic diagnosis and classification, with the ultimate goal of generating a canine glioma cancer genome atlas.
This effort was led by Drs. Jey Koehler, Andrew Miller, Ryan Miller, and Brian Porter
The Comparative Brain Tumor Consortium is collaborating with National Center for Advanced Translational Sciences to complete a high-throughput drug cell line screen. Three canine astrocytic glioma cell lines and two pediatric glioblastoma cell lines are included in the screen, which includes libraries of thousands of FDA-approved and investigational agents. Agents developed for oncology indications as well as non-oncology drugs were screened to support drug-repurposing hypotheses. Results will be made publically available.
This effort is being led by Dr. Matthew Hall.
The Comparative Brain Tumor Consortium is collaborating with National Center for Advanced Translational Sciences to complete whole exome sequencing on canine meningioma samples. Results will be published and made publicly available.
This effort is being led by Dr. Renee Chambers and Dr. Gregory Tawa.
In 2016, the NCI provided Administrative Supplements for P30 Cancer Center Support Grants (CCSG) to Support Research in Canine Immunotherapy via Collaboration of NCI-Designated Cancer Centers and Veterinary Medical Colleges. The supplemental funding was provided to promote expanding knowledge of canine cancer immunotherapy in the area of mutational load and neoantigens of a small number of specific canine organ-site tumors. Six tumor types were selected: B-cell lymphoma, melanoma, bladder cancer, osteosarcoma, glioma, and breast cancer (and their normal cell equivalents). The goal is to characterize the immunogenic mutational load (neoantigens that can strongly bind canine MHC type I antigens) in 25 cases of each of these tumors in advance of testing the suitability of canine models for the study of single and combination immunomodulating agents, molecularly targeted drugs or chemotherapeutics.
Results from the P30 awardees will be made publicly available.
The CBTC has opened a clinical trial in canine meningioma. Funding for the clinical trial has been generously provided by the American Kennel Club-Canine Health Foundation. We are evaluating Procaspase-3 Activator (PAC-1) in combination with Hydroxyurea for the treatment of canine meningioma. The trial is being led by Dr. Timothy Fan, and is open for recruitment at University of Pennsylvania and Virginia-Maryland Medical College. Dogs enrolled on study will also come to the National Cancer Institute to receive advanced imaging as part of the study.
Additional trial concepts are currently in development.
Sponsor: AKC-Canine Health Foundation
Study Numbers: 6-8 dogs anticipated
Participating Sites:
- Prior MRI with a diagnosis of meningioma
- Informed owner consent
- No prior radiation or chemotherapy
Status: Closed
Name: Molecular Combinatorial Therapy for Canine Malignant Gliomas
Dogs and humans are the only species in which primary brain tumors are common. In dogs, a type of malignant tumor called a glioma has a particularly poor prognosis, and there is no cure for it. In this clinical trial, we are testing the safety and effectiveness of molecularly targeted cytotoxins, which are chemotherapeutic drugs, delivered directly to the patient's brain tumor. The drugs used in this trial are designed to affect only cancerous cells, and not normal brain tissue.
Sponsor: National Institutes of Health
Participating Sites:
- Dogs of any age, breed, or sex > 3 and < 45 kg body weight
- Clinical signs of mild to moderate neurologic dysfunction referable to the brain
- MRI evidence of a single telencephalic intra-axial mass lesion consistent with a glioma
- No clinical or other diagnostic evidence of other significant systemic disease
MRI Consensus Document
The CBTC has published a MRI Consensus document in an effort to develop consensus recommendations for a standardized brain tumor imaging protocol for clinical trials using the canine model, such that: 1) multi-institutional studies can be performed with minimal inter-institutional variation, and 2) imaging protocols are consistent with human consensus recommendations to permit reliable translation to human clinical trials.
This effort is led by Dr. Rebecca Packer.
The National Cancer Institute Comparative Brain Tumor Consortium, Patient Outcomes Working Group, proposed a consensus document in support of standardized magnetic resonance imaging protocols for canine brain tumor clinical trials. This consensus document was published in 2018.
Earlier this year, the Comparative Brain Tumor Consortium (CBTC) sent out a survey to capture the landscape of canine brain tumors in North America. This project was conceived in order to better define the current standards amongst veterinarians in diagnosis, treatment and management of canine brain tumor patients to support future opportunities for translational and clinical studies. The survey was sent to all American College of Veterinary Internal Medicine (ACVIM) Board-certified neurologists. Results from the survey will be published later this year.