Background
The mission of the intramural National Cancer Institute’s Comparative Oncology Program (COP) is to utilize the canine cancer patient to advance knowledge of cancer biology and drug development to improve outcome for both dogs and humans. Advantages of studying spontaneous malignancies in pet dogs include similar body size, a shared home environment, an intact immune system, natural co-evolution of tumor and host stroma, and the application of comparable treatment platforms within veterinary and human medical care (i.e. chemotherapy, radiotherapy, surgery). To advance this mission, the COP has taken a specific research focus in osteosarcoma, linking canine clinical trial activities to laboratory efforts focused on comparative tumor biology and genomics.
Osteosarcoma (OS) is an aggressive pediatric/adolescent/young adult malignancy and is the most common malignant bone tumor in this patient population. Similarly, canine OS is an aggressive, naturally-occurring bone tumor of dogs that is >10x more common than in humans. Patients with metastatic or relapsed disease have dismal outcomes, with survival rates less than 30% despite aggressive salvage regimens. Standard of care for canine OS patients consists of amputation of the affected limb to achieve local tumor control, followed by systemic platinum-based chemotherapy. However, many clinical studies, including our own clinical trials that include over 400 canine patients, demonstrate that development of metastases, most often to the lung, occur in over 90% of canine patients within several months of diagnosis.
The DOG2 Project
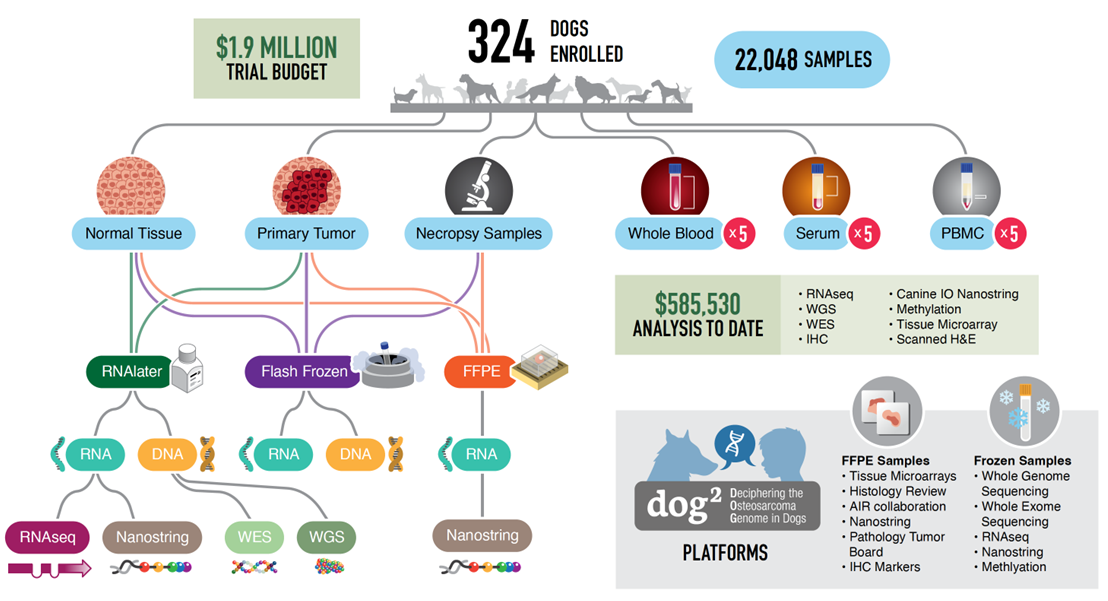
Key gaps in knowledge persist in the translatability of canine osteosarcoma (OS) to its human counterpart, inclusive of histologic, genomic and transcriptomic features. To address this gap in knowledge, we have created the DOG2 project (Decoding the Osteosarcoma Genome in Dogs (DOG2)). Under the NCI’s DOG2 project, we are addressing these gaps through a multi-omic approach utilizing samples collected from NCI-COTC clinical trials. 324 dogs with treatment naive osteosarcoma were enrolled in two simultaneous clinical trials, resulting in over 22,000 outcome-linked samples. A sample/data archive was created using LabMatrix to organize and track samples, images, and accompanying clinical and genomic/transcriptomic datasets.
Our program is leveraging the DOG2 outcome-linked OS biorepository to:
identify prognostic signatures for canine OS that could be translated to human patients
identify new druggable targets that can be biologically and experimentally validated
We have completed pathology review and nucleic isolation from primary OS tumors, normal tissues and metastatic samples. Analytical platforms include bulk and single-nuclei RNAseq, whole genome sequencing, whole exome sequencing, methylation profiling, multiplex IHC and Nanostring IO profiling.
The DOG2 biospecimen repository is enabling the creation of a clinical, genomic, and pathologic framework for canine OS, which will further enhance the translational value of the pet dog as a naturally-occurring animal model for humans.
DOG2 Specific Publications
- Transcriptional profiling of canine osteosarcoma identifies prognostic gene expression signatures with translational value for humans
- Patterns of metastatic progression and association with clinical outcomes in canine osteosarcoma: A necropsy study of 83 dogs
- Large Scale Comparative Deconvolution Analysis of the Canine and Human Osteosarcoma Tumor Microenvironment Uncovers Conserved Clinically Relevant Subtypes
- Clinical, pathologic and molecular findings in 2 Rottweiler littermates with appendicular osteosarcoma