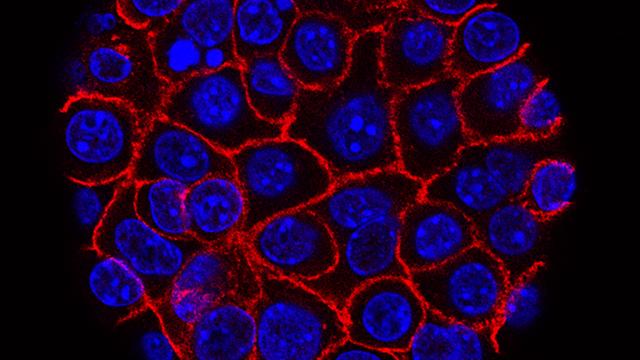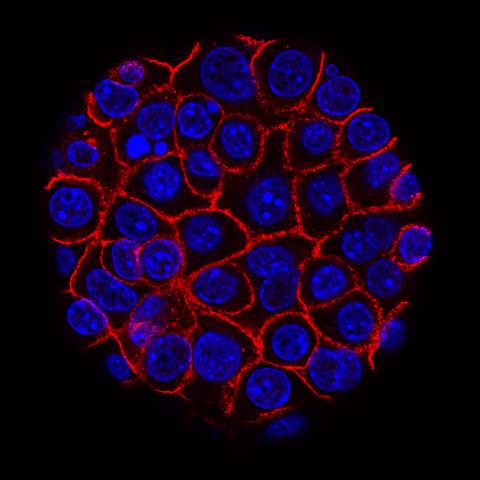
This image shows pancreatic cancer cells (nuclei in blue) growing as a sphere encased in membranes (red). By growing cancer cells in the lab, researchers can study factors that promote and prevent the formation of deadly tumors.
Image credit: NCI Visuals Online
Adults with advanced adenosquamous carcinoma of the pancreas (ASCP) may be eligible to participate in a new clinical trial at the NIH Clinical Center.
ASCP is a rare type of pancreatic cancer that is particularly aggressive. Christine Campo Alewine, M.D., Ph.D., Lasker Clinical Research Scholar in the Laboratory of Molecular Biology, is conducting a study to see if the drug Minnelide can effectively treat ASCP.
MYC is a family of regulator genes that drive ASCP cell growth. Minnelide works in part by inhibiting MYC. It rapidly releases an antitumor molecule into the bloodstream that slows cancer cell growth and induces cancer cell death. Investigators want to see if Minnelide alone can help to shrink ASCP tumors.
Clinicaltrials.gov identifier: NCT04896073
NCI Protocol ID: NCI-00-0-254
Official Title: A Phase II Trial of the Superenhancer Inhibitor Minnelide in Advanced Refractory Adenosquamous Carcinoma of the Pancreas (ASCP)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.