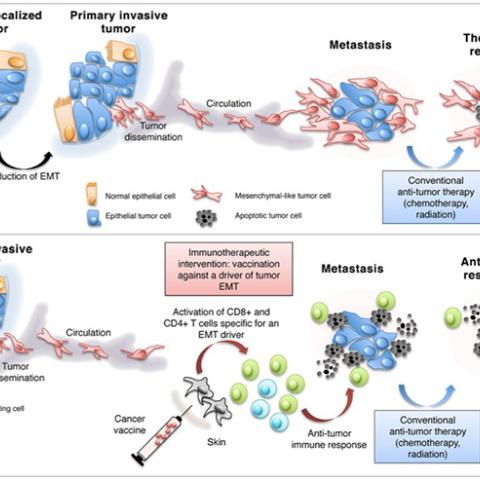
Targeting the epithelial-to-mesenchymal transition to block metastatic dissemination and alleviate resistance to therapy.
Patients with solid tumors that have spread or are not treatable by surgery may be eligible to participate in a new clinical trial at the NIH Clinical Center.
Marijo Bilusic, M.D., Ph.D., of the Genitourinary Malignancies Branch, is leading a clinical trial to test whether giving the MVA-BN-brachyury vaccine intravenously (IV) is safe for humans and has no intolerable side effects. The vaccine is based on a vector, or carrier, derived from vaccinia, the virus that causes smallpox. The modified vaccinia Ankara (MVA) version of the virus, developed by Bavarian Nordic (BN), has been weakened so that it cannot cause disease in humans. The MVA vector is encoded with brachyury, a protein highly expressed in many types of solid tumors. The vaccine also carries three molecules that stimulate the immune system to respond to the vaccine. When the vaccine is injected into a patient’s vein, the MVA vector releases the brachyury protein it is carrying. This, along with the three costimulatory molecules, activates T cells to attack tumor cells that also express brachyury. While on this study, patients with solid tumors that have spread, or cannot be treated surgically, will receive escalating doses of IV MVA-BN-brachyury vaccine every three weeks for a maximum of three IV injections. The goal of the study is to see whether IV MVA-BN-brachyury vaccine can shrink tumors and slow or prevent the spread of cancer to other parts of the body.
Clinicaltrials.gov identifier: NCT04134312
NCI Protocol ID: NCI-20-C-0001
Official Title: A Phase 1 Open Label Trial of Intravenous Administration of MVA-BN-Brachyury Vaccine in Patients With Advanced Cancer
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.