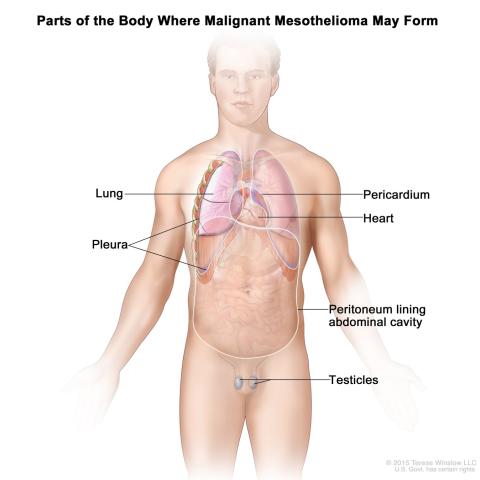
Malignant Mesothelioma
Image courtesy of NCI Visuals Online
Adults with mesothelioma of the lungs or abdomen may be eligible to participate in a clinical trial at the NIH Clinical Center.
Raffit Hassan, M.D., chief of the of the Thoracic and GI Malignancies Branch, along with a team of researchers, is researching a method to treat malignant mesothelioma. Mesothelioma is a rare, fast-growing cancer that forms in membranes that surround and protect the heart, lungs and abdomen. The cells of malignant mesothelioma express large amounts of mesothelin, a cancerous protein. LMB-100 is a drug that targets mesothelin. In other studies, when LMB-100 was given, it had some effect against cancer cells. However, after a while, patients became resistant to LMB-100. In this study, researchers want to see if injecting LMB-100 directly into a tumor will have greater anti-tumor effects, especially if given along with an immunotherapy drug, ipilimumab. Ipilimumab boosts the body’s immune response against cancer cells. Researchers want to identify the best and safest dose of LMB-100 and ipilimumab.
Clinicaltrials.gov identifier: NCT04840615
NCI Protocol ID: NCI-00-0-059
Official Title: Phase I Study of Intratumor Injection of Anti-Mesothelin Immunotoxin LMB-100 With Ipilimumab in Malignant Mesothelioma
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.