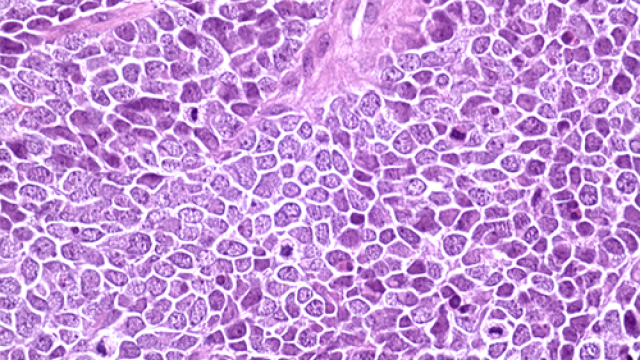
Image Source: J Immunother Cancer 2020 Feb;8(1):e000433. PMID: 32079617
People with metastatic, non-prostate genitourinary cancers may be eligible to participate in a clinical trial at the NIH Clinical Center.
Genitourinary (GU) cancers are common but difficult to treat with chemotherapy or immunotherapy alone. Andrea B. Apolo, M.D., Investigator and Lasker Clinical Research Scholar in the Genitourinary Malignancies Branch, is leading a study of two drugs that intensify the immune system’s attack on cancer cells. Study participants must have cancer that started in GU organs other than the prostate and has spread to other parts of the body. Patients receive NHS-IL12 and bintrafusp alfa (M7824) which affect the immune system in different ways. NHS-IL12 delivers a dose of IL-12, a naturally occurring protein, to dead or dying areas of a GU tumor and triggers the immune system to attack cancer cells. Bintrafusp alfa blocks signals that cancer cells use to avoid being destroyed by immune cells. Patients receive these drugs with or without a specialized radiation therapy to see if the combination leads to improved outcomes. Stereotactic body radiation therapy (SBRT) delivers intense, precise doses of radiation to cancer cells with a minimum of damage to healthy tissue. Other studies have shown that SBRT can work with immunotherapies to promote greater anticancer immune responses. Investigators want to see if NHS-IL12 and bintrafusp alfa together can fight metastatic, non-prostate GU tumors, with or without the help of SBRT.
Clinicaltrials.gov identifier: NCI-20-C-0012
NCI Protocol ID: NCT04235777
Official Title: A Phase I Study of Bintrafusp Alfa (M7824) and NHS-IL12 (M9241) Alone and in Combination With Stereotactic Body Radiation Therapy (SBRT) in Adults With Metastatic Non-Prostate Genitourinary Malignancies
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.