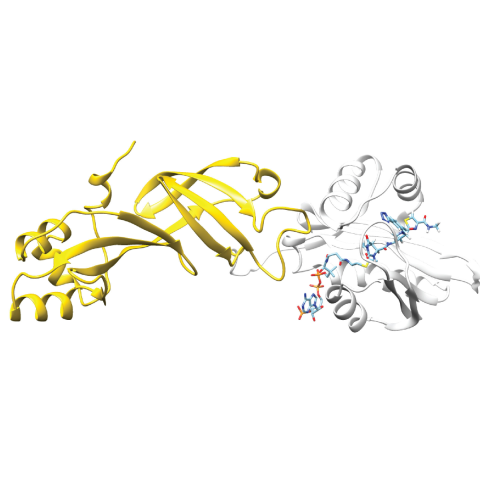
Researchers have developed a protein biosensor capable of detecting acetyl-CoA, a metabolite involved in cancer epigenetics. In their approach, a bioluminescent protein (Nanoluc, gold structure on the left) can bind to the fluorophore-bound acetyl-CoA binding protein (NAA50, white structure on the right). The bioluminescent signal generated by the pair is disrupted in the presence of acetyl-CoA.
CCR researchers have developed a new way to track a critical metabolite involved in normal and cancer cell metabolism, a small molecule called acetyl-CoA. The advance, published July 24, 2023, in the Journal of the American Chemical Society, will help scientists study how this molecule influences gene expression with unprecedented detail. This result could eventually lead to new diagnostic cancer tests.
Acetyl-CoA is a metabolite found in every cell of the human body. While it plays many important roles, it is believed to help turn genes on and off by modifying chromatin in the nucleus of cells (a process called histone acetylation). However, a lack of methods to study acetyl-CoA in the nucleus has made it challenging to understand the phenomenon in greater detail.
One possible way to track and quantify acetyl-CoA within live cells would be a technique called BRET, or bioluminescence resonance energy transfer. With this imaging technique, researchers identify a protein that binds to the metabolite of interest and engineer it to emit light. When the engineered protein binds with the metabolite, the light signal is turned off — providing a clear indication that the metabolite is present, and in what quantity based on the luminescence.
“However, we don’t know of any proteins capable of selectively binding to acetyl-CoA and turning that binding event into an optical signal. Our study attempts to solve this piece of the puzzle,” explains Jordan Meier, Ph.D., a Senior Investigator in the Chemical Biology Laboratory.
To do so, Meier’s team, led by postbaccalaureate fellows Whitney Lieberman and Zachary Brown, partnered with researchers at Temple University in Philadelphia, PA, including experts in acetyl-CoA metabolomics, Nathaniel Snyder’s group.
“We used an approach called chemoproteomics that allowed us to ‘sift’ through a large number of proteins that human cells make. We were able to find one that can bind specifically to acetyl-CoA,” explains Meier.
The research team identified 23 proteins of interest and focused on one particular protein, NAA50. Using protein engineering, they turned it into a biosensor that glows. When the engineered version of NAA50 encounters acetyl-CoA in the cell nucleus, its light is shut off. In this way, researchers can track the presence and quantity of acetyl-CoA in live cells in real-time. However, this technique is currently an indirect means of tracking acetyl-CoA, and more work is needed to use this technique to image the metabolite directly.
“The proteins discovered in this study set the stage for finding approaches that will enable direct fluorescent imaging of acetyl-CoA concentrations, which has been a ‘holy grail’ of the field of [cell] metabolism,” says Meier.
Additionally, enzymes that interact with acetyl-CoA have been linked to cancer, meaning that better tools for studying acetyl-CoA could also help researchers develop drugs targeting these processes and potentially lead to additional ways of detecting and diagnosing cancer.
“Our future research will focus on improving the selectivity of acetyl-CoA sensing, leveraging the proteins discovered in this study for cellular imaging, and also seeing if they may be exploited for fluorescent detection,” says Meier.