Clinical Trials
NCI initiative aims to boost CAR T-cell therapy clinical trials
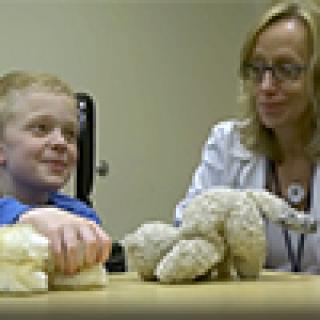
Researchers at the Center for Cancer Research are part of a new NCI initiative to manufacture CAR T-cell therapies for clinical trials being conducted at multiple hospitals. Nirali N. Shah, M.D., Lasker Clinical Research Scholar in the Pediatric Oncology Branch, is a co-lead investigator on the first trial of this initiative that is testing a CAR T-cell therapy designed to target a protein on cancer cells called CD33 in children and young adults with advanced forms of acute myeloid leukemia.
Read MoreThe discovery of selumetinib for children with NF1
When Brigitte Widemann, M.D., Chief of the Pediatric Oncology Branch (POB), saw selumetinib shrinking the first child’s tumor, she couldn’t believe her eyes. Then, when she saw the second patient’s tumor shrinking, “I thought, oh my gosh, it’s working!” she recalls. Over 30 years of NCI-led and NCI-supported research culminated in clinical trials of selumetinib for kids with neurofibromatosis type 1 (NF1) and associated tumors called plexiform neurofibromas (PNs). With Food and Drug Administration (FDA) approval, selumetinib is now the first effective treatment for kids with PNs that can't be removed by surgery.
Read MoreFDA approves first therapy for children with neurofibromatosis type 1
The Food and Drug Administration (FDA) has approved selumetinib (Koselugo) to treat children with neurofibromatosis type 1 (NF1) and tumors called plexiform neurofibromas. The approval was based on results from a clinical trial, designed and led by Brigitte Widemann, M.D., Chief of the Pediatric Oncology Branch (POB), and Andrea M. Gross, M.D., Assistant Research Physician in POB, that showed the drug shrank neurofibromas in 70 percent of patients. For many children in the trial, treatment with selumetinib also had clinical benefit, improving their pain, function and quality of life.
Read MoreCCR researchers show selumetinib shrinks tumors in children with NF1
Findings from a phase 2 clinical trial show that the drug selumetinib improves outcomes for children with the genetic disorder neurofibromatosis type 1 (NF1). In the trial, selumetinib shrank the inoperable tumors that develop with NF1 called plexiform neurofibromas, and children experienced reduced pain, improved function, and better overall quality of life after receiving the treatment. This trial was led by Brigitte Widemann, M.D., Chief of the Pediatric Oncology Branch (POB), and Andrea M. Gross, M.D., Assistant Research Physician in POB, and the results were published March 18, 2020, in the New England Journal of Medicine.
Read MoreNew Milestones publication now available
Every year, the Center for Cancer Research makes remarkable contributions to the understanding, detection, treatment and prevention of cancer. This issue of our annual publication, Milestones, features some of the most impactful science conducted in the past year in CCR. These advances include new insights into how genomes are organized and how DNA and RNA function in cells, how cellular processes and signaling events function in healthy cells and how they are affected in cancer. Other major discoveries this year include how cancers become metastatic and what drives the proliferation of cancer cells.
Read MoreCCR researchers show testing with combined biopsy method improves prostate cancer diagnosis
A method of testing for prostate cancer developed at the Center for Cancer Research leads to more accurate diagnosis and prediction of the course of the disease, according to a new study published in the New England Journal of Medicine. Peter Pinto, M.D., Investigator in the Urologic Oncology Branch, led the study and says this method, which combines systematic biopsy, the current primary diagnostic approach, with MRI-targeted biopsy, is poised to greatly improve prostate cancer diagnosis, thereby reducing the risk of both overtreatment and undertreatment of the disease. To see all open prostate cancer trials, click here.
Read MoreClinical trial studies combination therapy for mesothelin-expressing solid tumors
A clinical trial at the NIH Clinical Center is evaluating a combination therapy for mesothelin-expressing solid tumors. Mesothelin is a protein found on the surface of certain types of normal cells and cancer cells. This trial is studying the effect of LMB-100, a man-made protein attracted to mesothelin, with a drug commonly used to treat certain types of arthritis and colitis.
Read MoreJennifer Brudno and James Kochenderfer discuss reduced side effects of remodeled CAR T-cell therapy
Jennifer Brudno, M.D., Assistant Research Physician, and James Kochenderfer, M.D., Investigator, both of the Surgery Branch, discussed their ongoing work to remodel CAR T cells to create a safer, more effective therapy in a recent Cancer Currents blog post. They share that since they tweaked the design of their original CAR T cells, the new therapy caused far fewer neurologic side effects than the original therapy did in an earlier trial but was equally effective.
Read MoreJames N. Kochenderfer receives Top Ten Clinical Research Achievement Award
James N. Kochenderfer, M.D., Investigator in the Surgery Branch, has been named a Top Ten Clinical Research Achievement Awardee by the Clinical Research Forum. Dr. Kochenderfer received the award for “Development of CAR T-Cell Therapy for Myeloma.”
Read MoreClinical trial tests combination immunotherapy for genitourinary tumors
Andrea B. Apolo, M.D., of the Genitourinary Malignancies Branch, is leading a study to recruit patients with each of the nearly 40 rare genitourinary malignancies and treat them with a combination of chemotherapy and immunotherapy drugs.
Read More