Clinical Trials
Clinical trial to test drug for cancer patients with weakened immune systems
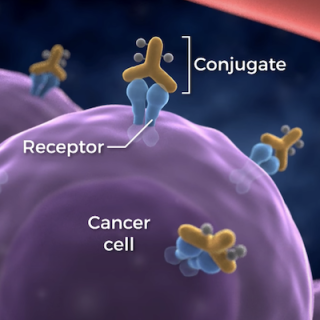
Cancer survivors age 60 and older have weakened immune systems, often caused by cancer treatments. Investigators want to see if a drug, NT-I7, is able to boost the response to vaccines in patients who otherwise may not be able to respond.
Read MoreJapan approves photoimmunotherapy for head and neck cancer
Promising clinical trials have led to the regulatory approval of the Bioblade® Laser System and Akalux® IV Infusion 250mg in Japan. This device and drug combination was developed under an investigational treatment platform based on a cancer therapy called photoimmunotherapy.
Read MoreNew clinical trial studies stem cell transplant for primary immunodeficiency diseases
Dennis D. Hickstein, M.D., Senior Investigator in the Immune Deficiency Cellular Therapy Program is leading a study that uses new DNA technology that speeds up the process of screening for primary immunodeficiency diseases (PIDs) and finding an acceptable donor match for hematopoietic stem cell transplant (HSCT).
Read MoreClinical trial studies therapy for people with prostate or kidney cancer
James L. Gulley, M.D., Ph.D., Chief of the Genitourinary Malignancies Branch, is leading the National Cancer Institute’s participation in a clinical trial of an experimental drug called JNJ-63898081. The goal of this multicenter study is to find out if the drug is safe to use in humans and to determine the optimal dose for the second phase of the study.
Read MoreCombination therapy tested in clinical trial for metastatic genitourinary cancers
Genitourinary cancers are common but difficult to treat with chemotherapy or immunotherapy alone. A new clinical trial studies two drugs that intensify the immune system’s attack on cancer cells.
Read MorePhase I CAR T-cell therapy leads to years-long remissions in relapsed B-cell lymphoma patients
In a Journal of Clinical Oncology article, results of a phase I trial by CCR investigators show that CAR T-cell therapy can result in long-lasting remissions in patients with certain relapsed B-cell lymphomas. Many who had life expectancies of only six months or less during the clinical trial of the therapy, which spanned from 2009 to 2015, remain in complete remission.
Read MoreClinical trial tests immunotherapy combination for advanced HPV-associated cancers
Human papillomavirus (HPV) is the most common sexually transmitted infection. More than 30,000 cases of HPV+ cancers occur every year in the United States. CCR investigators are leading a study using a combination of 3 immunotherapy drugs to treat HPV+ cancers.
Read MoreClinical trial tests vaccine for late-stage HPV-linked tumors
The human papillomavirus (HPV) has been linked to many kinds of cancer, including cervical, uterine, vaginal, penile and oropharyngeal. For those who develop advanced HPV-linked cancer, the NIH Clinical Center has a clinical trial open to test a vaccine with and without checkpoint inhibitors to see if this treatment approach can stop tumor growth.
Read MoreLymphoma therapy drug tested as early treatment for chronic graft-versus-host disease
cGvHD can occur after a person has had a stem cell or bone marrow transplant. In some cases, the donated bone marrow/stem cells view the host's body as foreign and start to attack it. cGvHD can occur at any time after a transplant, but it's more common after the marrow/stem cells have created a new immune system in the host's body. A clinical trial is studying the lymphoma therapy drug ibrutinib to see if early treatment can prevent the most severe symptoms of cGvHD.
Read MoreCombination therapy for solid tumors and small-cell cancers studied in new clinical trial
A clinical trial of a drug combination to treat solid tumors and small-cell cancers is being conducted at the NIH Clinical Center. PARP inhibitors can work better when combined with chemotherapy, such combinations can be too toxic, so this study uses a new kind of chemotherapy called PLX038 and combines it with a PARP inhibitor rucaparib to see if the combination of PLX038 and rucaparib can safely shrink solid tumors and small-cell cancers.
Read More