Clinical Trials
Clinical trial targets cell surface protein GPC3 to treat advanced hepatocellular carcinoma
Tim F. Greten, M.D., Deputy Chief of the Thoracic and GI Malignancies Branch, is leading a study of advanced hepatocellular carcinoma, a common type of liver cancer with a poor prognosis. The research uses CAR T-cell therapy to target GPC3-positive tumor cells while avoiding healthy tissue.
Read MoreIbrutinib improves survival for younger people with diffuse large B-cell lymphoma
New evidence suggests that adding the drug ibrutinib to a standard chemotherapy regimen can improve how long some younger people with a specific form of diffuse large B-cell lymphoma live. The findings come from a new analysis led by Louis M. Staudt, M.D., Ph.D., Chief of the Lymphoid Malignancies Branch, of a previous phase III clinical trial.
Read MoreClinical trial studies breast cancer drug abemaciclib as a therapy for Kaposi sarcoma
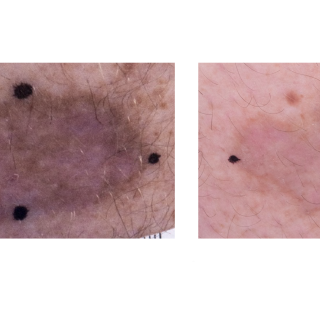
Kaposi sarcoma (KS) is a rare cancer that causes patches of abnormal tissue to develop in different regions of the body, and lesions in the lungs, liver, or digestive tract can be life-threatening. Investigators are studying abemaciclib, a drug used for people with breast cancer, to see if it can positively impact those with KS.
Read MoreClinical trial evaluates new combination therapy for virus-associated malignancies
Several viruses can cause cancer and less toxic and more effective treatments are urgently needed for these cancers. Kathryn A. Lurain, M.D., M.P.H., Assistant Research Physician in the HIV and AIDS Malignancy Branch, is leading a trial of a combination treatment for virus-associated malignancies.
Read MoreClinical trial studies targeted therapy for PARP-resistant tumors and chemotherapy-resistant small cell lung cancer
Anish Thomas, M.B.B.S., M.D., Lasker Clinical Research Scholar in the Developmental Therapeutics Branch, is leading a study using a berzosertib-sacituzumab govitecan combination to target ATR, a key protein involved in an alternative DNA repair pathway used by chemotherapy and PARP inhibitor-resistant cancer cells.
Read MoreImmunotherapy studied in clinical trial for glioblastoma or gliosarcoma
Adults with glioblastoma (GBM) or gliosarcoma may be eligible to participate in a clinical trial at the NIH Clinical Center. GBM and gliosarcoma tumors can suppress the immune system so immune cells cannot work effectively within the brain. Mark Gilbert, M.D., Chief of the Neuro-Oncology Branch, is leading a study to see if this suppression can be reversed with immune checkpoint inhibitors.
Read MoreClinical trial evaluates reduced dosing of cyclophosphamide to prevent severe acute graft-versus-host disease
Christopher G. Kanakry, M.D., Lasker Clinical Research Scholar in the Experimental Transplantation and Immunotherapy Branch, is leading a study that may help people with cancers that begin in blood-forming tissue who are at high risk for graft-versus-host disease (GVHD) following bone marrow transplant. This study will evaluate if reduced dosing of cyclophosphamide is effective in preventing severe acute GVHD.
Read MoreClinical trial evaluates the effect of Minnelide on advanced pancreatic cancer
Adenosquamous carcinoma of the pancreas (ASCP) is a rare type of pancreatic cancer that is particularly aggressive. Christine Campo Alewine, M.D., Ph.D., Lasker Clinical Research Scholar in the Laboratory of Molecular Biology, is conducting a study to see if the drug Minnelide can effectively treat ASCP.
Read MoreClinical trial studies surgical treatment for adults with peritoneal carcinomatosis
Andrew M. Blakely, M.D., Assistant Research Physician in the Surgical Oncology Program, is leading a surgical study for adults with peritoneal carcinomatosis that tests two chemotherapy treatments, called HIPEC, simulated in a tissue-testing platform.
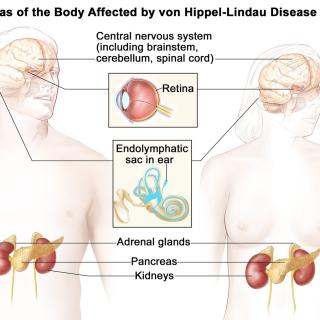
Read MoreFDA approves belzutifan, first drug for cancers associated with von Hippel-Lindau disease
On August 13, 2021, the Food and Drug Administration approved belzutifan, a new drug for adult patients with von Hippel-Lindau (VHL) disease-associated renal cell carcinoma (RCC), central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors, not requiring immediate surgery.
Ramaprasad Srinivasan, M.D., Ph.D., Investigator in the Urologic Oncology Branch (UOB), designed the ongoing study and played a key leadership role as the principal investigator on the cooperative research and development agreement under which NCI served as a site in the study. Belzutifan is now the first and only approved systemic therapy for certain patients with VHL-associated RCC.
VHL disease is a rare, inherited disorder that causes tumors and cysts to grow in certain parts of the body. Patients with this disease have an increased risk of certain types of cancer, especially kidney cancer and pancreatic cancer. The VHL gene was originally identified by Marston Linehan, M.D., and colleagues in the UOB in the 1990s, and the group continues to define the methods for clinical management of VHL disease.
Read More